Juntendo Educational Corporation
Cervical cancer treatment with iPS cell-derived resident memory T cells ― Derived from a healthy person Reduced rejection through genome editing technology ―
……
Graduate School of Medicine Yoshiki Furukawa, Assistant Professor Midori Ishii, Professor Miki Ando of Hematology, Juntendo University Graduate School of Medicine, Professor Jun Ando of Cell Therapy and Blood Transfusion, Professor Yasuhisa Terao of Obstetrics and Gynecology, and Stem Cell Biology and Regeneration, Stanford University School of Medicine. A joint research group led by Professor Hiromitsu Nakauchi of the Institute of Medical Research is conducting genome editing on iPS cells established from healthy individuals to generate human papillomavirus (*1)-specific cytotoxic T cells from the iPS cells. (CTL) (*2) has been shown to be able to strongly suppress cervical cancer without being rejected by the patient’s immune cells. Furthermore, it was revealed that iPS cell-derived CTL (*3) has high cytotoxic activity because it contains a large amount of tissue resident memory T cells (*4), which shows that it has a promising potential for treating refractory cervical cancer. This demonstrated the potential for a novel treatment. These results will provide basic data for an investigator-initiated clinical trial of iPS cell-derived CTL therapy for cervical cancer, which is scheduled to begin next summer.
This paper was published in advance in the online version of the Cell-based academic journal “Cell Reports Medicine” on December 12, 2023 (US Eastern Time).
◆Key points of this research result
Succeeded in creating CTLs with reduced alloimmune reaction by genome editing iPS cells derived from healthy individuals
It was discovered that because the iPS cell-derived CTL contains abundant tissue resident memory T cells, it can suppress cervical cancer more strongly than the original CTL.
◆Background
Cervical cancer is caused by human papillomavirus (HPV) infection. While the number of patients and mortality rates are decreasing in developed countries due to the spread of vaccines, the trend continues to increase in Japan, with more than 10,000 new cases of cervical cancer being diagnosed each year, and approximately 3,000 cases of cervical cancer. Dead. In Japan, the vaccination rate for cervical cancer remains low at less than 15%, and the increase in the number of cervical cancer cases among people in their 20s and 30s is a particularly serious problem. Cervical cancer is called the mother killer, and as it progresses extremely rapidly and has a poor prognosis in young women who are married, pregnant, and raising children, it is necessary to develop new effective treatments. In 2020, the research group successfully developed iPSC-derived HPV antigen-specific CTL (HPV-CTL) against cervical cancer, and
demonstrated its sustained and powerful antitumor effect. However, the production of CTLs from the blood of cervical cancer patients undergoing anticancer drug treatment is difficult and time-consuming, and the production costs are high, which has been a challenge for practical application. On the other hand, while using allogeneic iPS cells established from CTLs from healthy individuals solves the above problems, it also causes an alloimmune reaction from the patient’s immune cells, which reduces the antitumor effect. Therefore, in order to solve this problem, the research group attempted to develop allogeneic HPV-CTL derived from healthy individuals with genome-edited HLA (*6) class I using CRISPR/Cas9 technology (*5).
◆Contents
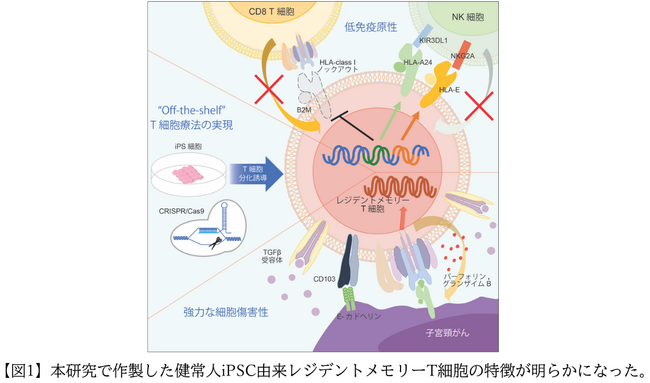
In this study, we first induced HPV-CTL from the peripheral blood of healthy individuals and then established iPS cells. Next, the iPS cells’ HLA class I antigens were edited to prevent them from being attacked by the patient’s immune cells. For genome editing, we decided to use the CRISPR/Cas9 two-step editing method, which has fewer off-target effects (*5), in consideration of safety in order to generate cells for clinical use. First, by knocking out the B2M gene, which is important for the presence of HLA class I molecules, and eliminating the expression of HLA class I antigens, we prevented the patient from being attacked by immune T cells. However, this alone would cause the patient’s NK cells (natural killer cells) to attack cells that do not express HLA class I antigens, so we forced the patient to express some class I molecules to avoid the next attack. By expressing both HLA-E and HLA-A24 molecules, which are known to have inhibitory bonds with NK cells, we were able to effectively suppress NK cell attacks. After inducing CTL differentiation from genome-edited iPS cells, we analyzed the alloimmune response and were able to prove that HPV-CTL derived from genome-edited iPS cells is not rejected by T cells and can also evade attacks from NK cells. Ta. Furthermore, we confirmed whether genome editing would reduce the antitumor effect against cervical cancer. As a result, the genome-edited iPS
cell-derived HPV-CTL was able to strongly suppress the growth of cervical cancer, regardless of the presence or absence of editing. It exerted a stronger antitumor effect in a shorter period of time than the original HPV-CTL derived from peripheral blood, and also showed a long-term survival prolongation effect.
Therefore, we conducted single cell analysis (* 7) and compared the gene expression. As a result, iPS cell-derived HPV-CTL has more genes related to cytotoxic activity (IFNG, PRF1, GZMB) and genes related to tissue resident memory T cells (ITGAE, CD69, TGFBR1) than peripheral blood-derived HPV-CTL. We found that the expression level of was significantly higher. Analysis by flow cytometry also showed that iPS cell-derived HPV-CTL contains abundant tissue-resident memory T cells. Functional analysis demonstrated that iPS cell-derived HPV-CTL significantly increased CD103 expression level through TGFβ signaling (*8), which resulted in enhanced HPV antigen-specific cytotoxic activity.
Based on the above, in this study, we used iPS cell technology and genome editing technology to successfully create HPV-CTL that can evade attack by patient immune cells from iPS cells derived from healthy individuals. As a result of its rich cell content, it was revealed that it has strong cytotoxic activity against cervical cancer. The successful development of next-generation T cell therapy that can reduce alloimmune reactions and enhance T cell function suggests that HPV-CTL could be a revolutionary new treatment for cervical cancer. (Figure 1).
[Image
This research was supported by AMED (JP19bm0404032, JP19be0404011, and JP21bk0104117) and JSPS KAKENHI (18K07273) and was conducted in collaboration with multiple institutions. We would like to express our deepest gratitude to everyone who cooperated with this research.
